Summary of Contents for Philips HeartStart M5066A
- Page 1 H e a r t S t a r t D e f i b r i l l a t o r O W N E R ’ S M A N U A L Guide to Set Up, Operation, Maintenance, and Accessories M5066A Edition 7...
- Page 2 Intentionally blank.
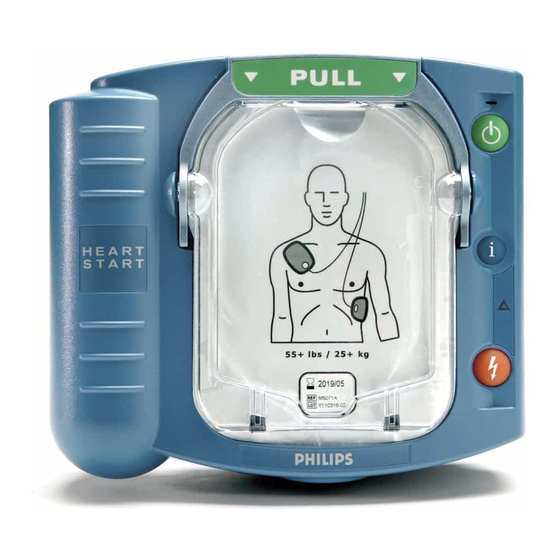
- Page 3 The HeartStart Defibrillator M5066A Pads Cartridge Handle. Pull the handle to turn on the HeartStart and remove the cartridge’s hard cover. Ready Light. This green light tells you the readiness of the HeartStart. Blinking: standby mode (ready for use)
- Page 4 Intentionally blank.
- Page 5 HeartStart Defibrillator M5066A QUICK REFERENCE...
- Page 6 Intentionally blank.
- Page 7 HeartStart M5066A Automated External Defibrillator O W N E R ’ S M A N U A L Edition 7 IMPORTANT NOTE: It is important to understand that survival rates for sudden cardiac arrest are directly related to how soon victims receive treatment. For every minute of delay, the chance of survival declines by 7% to 10%.
- Page 8 Intentionally blank.
- Page 9 About this edition The information in this guide applies to the model M5066A HeartStart Defibrillator. Its technical contents apply to all models in the HeartStart HS1 family of defibrillators, including the HeartStart, the HeartStart OnSite, and the HeartStart First Aid Defibrillator. This information is subject to change.
- Page 10 Intentionally blank.
-
Page 11: Table Of Contents
CONTENTS 1 Introduction to the HeartStart Description ... 1-1 Sudden Cardiac Arrest ... 1-1 Indications for Use ... 1-1 Training and practice ... 1-2 National and local requirements ... 1-2 For more information ... 1-2 2 Setting up the HeartStart Package contents ... - Page 12 APPENDICES A Accessories for the HeartStart B Glossary of terms C Glossary of symbols/controls D Warnings and precautions E Technical information Configuration G Testing and troubleshooting H Additional technical information required for European conformity HEARTSTART M5066A...
-
Page 13: Description
Introduction to the HeartStart Description The HeartStart Defibrillator M5066A is part of the Philips HeartStart HS1 family of defibrillators. Small, lightweight, and battery powered, it is designed for simple and reliable operation. Sudden Cardiac Arrest The HeartStart is used to treat the most common causes of sudden cardiac arrest (SCA), including ventricular fibrillation (VF). -
Page 14: Training And Practice
Contact your Philips representative, or visit us on-line at www.medical.philips.com, for information about training programs in your area. NOTE: Training accessories are available from Philips for practicing use of the HeartStart. See Appendix A for information on ordering accessories. National and local requirements Check with your local health department to see if there are any national or local requirements about owning and using a defibrillator. -
Page 15: Package Contents
Setting up the HeartStart Package contents Check the contents of the HeartStart Defibrillator M5066A box to be sure it contains: • 1 HeartStart Defibrillator • 1 battery M5070A • 1 Adult SMART Pads Cartridge M5071A, containing one set of adhesive defibrillation pads •... - Page 16 * As long as a battery is installed, turning the HeartStart “off” puts it into standby mode, HEARTSTART M5066A Insert the cartridge into the cartridge well on the front of the defibrillator. It should click into place when properly seated.
- Page 17 Place the defibrillator in the carry case, pressing it firmly into place. Insert the Quick Reference Guide, face up, in the clear plastic window on the inside of the case. If you purchased a spare SMART Pads Cartridge or an Infant/Child Pads Cartridge, place it in the storage area in the case.
-
Page 18: Recommended Accessories
HeartStart include: Philips has a Fast Response Kit with all these items. See Appendix A for details. If you may need to defibrillate an infant or a child under 25 kg (55 pounds) or 8 years old, it is recommended that you order the Infant/Child SMART Pads Cartridge, available separately. -
Page 19: Overview
Using the HeartStart IMPORTANT NOTE: Be sure to read the Reminders section at the end of this chapter as well as the warnings and precautions in Appendix D. Overview If you think someone is in SCA, act quickly and calmly. If someone else is available, ask him or her to call for emergency medical assistance while you get the HeartStart. -
Page 20: Step 1: Pull The Green Handle
The HeartStart starts by directing you to remove all clothes from the patient’s chest. If necessary, rip or cut off the clothing to bare the person’s chest * You can also turn on the HeartStart Defibrillator by pressing the green On/Off button. HEARTSTART M5066A... -
Page 21: Step 2: Place The Pads
STEP 2: PLACE the pads Pull the tab at the top of the pads cartridge to peel off the film seal. Inside are two adhesive pads on a plastic liner. Remove the pads from the cartridge. Peel one pad off the liner. Place the pad on the patient’s bare skin, exactly as shown in the picture on the pad. -
Page 22: Step 3: Press The Shock Button
30 seconds of the patient care (If the Infant/Child SMART Pads Cartridge is i-button in this situation; however, the default setting can be revised by your Medical Director using Philips software available separately. See Appendix F for more information. begins flashing as a reminder. -
Page 23: Treating Infants And Children
CPR Coaching. Place the pads exactly as shown on the illustration on the pads. * Philips recommends that the HeartStart be stored with an adult pads cartridge installed, as pediatric cardiac arrest is not common. † This lower energy level may not be effective for treating an adult. -
Page 24: When Emergency Medical Services Arrive
HeartStart to service, to be sure it is ready for use. * See Chapter 4, “After using the HeartStart” for details about data storage. HEARTSTART M5066A DO NOT DELAY TREATMENT. Remove all clothing from the torso, to bare both the chest and the back. - Page 25 • Reminders Remove any medicine patches and residual adhesive from the patient’s chest before applying the pads. • Do not allow the pads to contact other electrodes or metal parts that are in contact with the patient. • Avoid placing the pads directly over an implanted pacemaker or defibrillator. A noticeable lump with a surgical scar should indicate the position of an implanted device.
- Page 26 Notes HEARTSTART M5066A...
-
Page 27: After Each Use
After each use Check the outside of the HeartStart for signs of damage, dirt, or contamination. If you see signs of damage, contact Philips for technical support. If the defibrillator is dirty or contaminated, clean it according to the guidelines in Chapter 5, “Maintaining the HeartStart.”... - Page 28 * The HeartStart automatically stores information about its last clinical use in its internal † If ECG recordings from a previous use have not been erased, the maximum time for new HEARTSTART M5066A Details about data transfer and timing are provided in ECG recordings (a maximum of 15 minutes following pads application the HeartStart’s status (entire incident)
-
Page 29: Routine Maintenance
WARNING: Electrical shock hazard. Do not open the HeartStart, remove its covers, or attempt repair. There are no user-serviceable components in the HeartStart. If repair is required, return the HeartStart to Philips for service. Reminders: • Do not leave the HeartStart without a pads cartridge installed; the defibrillator will start chirping and the i-button will start flashing. -
Page 30: Cleaning The Heartstart
The HeartStart’s green Ready light is your guide to knowing if the defibrillator is ready for use. More detailed testing and troubleshooting information is available in Appendix G. HEARTSTART M5066A Do not use isopropyl (rubbing) alcohol, strong solvents such as acetone or acetone-based cleaners, abrasive materials, or enzymatic cleaners to clean your HeartStart. - Page 31 Accessories for the HeartStart Accessories for the HeartStart Defibrillator available separately from your Philips representative or on-line at www.medical.philips.com/heartstart include: • Battery (spare recommended) [REF: M5070A] • Pads • Adult SMART Pads Cartridge (spare recommended) [REF: M5071A] • Infant/Child SMART Pads Cartridge [REF: M5072A] •...
- Page 32 • HEARTSTART M5066A Training • Adult Training Pads Cartridge [REF: M5073A] • Adult Training Replacement Pads [REF: M5093A] • Adult Pads Placement Guide [REF: M5090A] • Infant/Child Training Pads Cartridge [REF: M5074A] • Infant/Child Training Replacement Pads [REF: M5094A] •...
- Page 33 Glossary of terms The terms listed in this Glossary are defined in the context of the Philips HeartStart Defibrillator and its use. Automated external defibrillator (a semi-automatic defibrillator). AED mode The standard treatment mode for the HeartStart Defibrillator. It provides voice instructions guiding the rescuer through applying the adhesive pads, waiting for rhythm analysis, and delivering a shock if needed.
- Page 34 A suite of data management software applications for use by trained personnel to review and analyze HeartStart Defibrillator patient use and by authorized personnel to alter HeartStart configuration. Information is available from Philips Medical Systems on the internet at http://www.medical.philips.com/goto/ eventreview.
- Page 35 On/Off button stops the battery insertion self-test that automatically runs when a battery is inserted. pads See “SMART pads.” patient care pause A defined pause to allow patient assessment, treatment, and/or CPR. See “NSA pause” and “protocol pause.” periodic self-tests Daily, weekly, and monthly tests automatically conducted by the HeartStart Defibrillator when it is in its standby mode.
- Page 36 READY light. standard NSA pause See “NSA pause.” sudden cardiac arrest The sudden stopping of the heart’s pumping rhythm, accompanied by loss of (SCA) consciousness, absence of respiration, and lack of a pulse. waveform See “SMART biphasic waveform.” HEARTSTART M5066A...
- Page 37 Glossary of symbols/controls symbol description Pads cartridge handle. Green. Pulling the handle turns on P U LL the defibrillator and opens pads cartridge for use. Refer to operating instructions. On/Off button. Green. Pressing the On/Off button when the defibrillator is in standby mode turns the defibrillator on;...
- Page 38 Class 9 miscellaneous dangerous goods. (Symbol required on outer packaging by freight carrier regulations to identify shipments containing lithium batteries.) Install the battery in the defibrillator before the date (MM-YYYY) shown on the associated label. Do not expose to moisture. Handle with care. HEARTSTART M5066A...
- Page 39 symbol description This side up. Transportation requirements (refer to associated thermometer symbol). Storage requirements (refer to associated thermometer symbol). Environmental (temperature and relative humidity) requirements. These pads are disposable and are for single patient use only. Cartridge contents: one set of two defibrillation pads. Store the pads at temperatures between 0°...
- Page 40 Serial number. Lot number. Federal law (USA) restricts this device to sale by or on the order of a physician. Dispose of in accordance with your country's requirements. Printed on recycled paper. HEARTSTART M5066A...
-
Page 41: Warnings And Precautions
HeartStart, loss of data stored in the HeartStart, or less chance of successful defibrillation. NOTE: The HeartStart Defibrillator is designed to be used only with Philips- approved accessories. The HeartStart may perform improperly if non- approved accessories are used. - Page 42 Before delivering a shock, it is important to disconnect the patient from other medical electrical equipment, such as blood-flow meters, that may not incorporate defibrillation protection. In addition, make sure the pads are not in contact with metal objects such as a bed frame or stretcher. HEARTSTART M5066A...
- Page 43 Technical information HeartStart Defibrillator specifications The specifications provided in the following tables are nominal values. Additional information can be found in the Technical Reference Manual for HeartStart Automated External Defibrillators, located online at www.medical.philips.com. Physical category specifications size 2.80” H x 7.40” D x 8.30” W (7.1cm H x 19cm D x 21cm W).
- Page 44 Shock button: orange, flashes when the defibrillator is charged and ready to deliver a shock. audio speaker Provides voice prompts and warning tones during normal use. beeper Provides chirps when troubleshooting is needed. HEARTSTART M5066A...
- Page 45 Defibrillation Waveform category specifications waveform parameters Biphasic truncated exponential. Waveform parameters are automatically adjusted as a function of patient defibrillation impedance. In the diagram at left, D is the duration of phase 1 and E is the duration of phase 2 of the waveform, F is the interphase delay (500 µs), and Ip is the peak current.
- Page 46 Via adhesive pads placed in the anterior-anterior (Lead II) position. delivery vector infant/child shock Via adhesive pads typically placed in the anterior-posterior position. delivery vector HEARTSTART M5066A energy dose newborn 1 year 2 − 3 years 4 − 5 years 6 −...
- Page 47 ECG analysis system category specifications function Evaluates impedance of adhesive pads for proper contact with the patient’s skin, and evaluates the ECG rhythm and signal quality to determine if a shock is appropriate. shockable rhythms Ventricular fibrillation (VF) and some ventricular tachycardias associated with a lack of circulation, including ventricular flutter and polymorphic ventricular tachycardia (VT).
- Page 48 From Philips Medical Systems Heartstream ECG rhythm databases. b. American Heart Association (AHA) AED Task Force, Subcommittee on AED Safety & Efficacy. Automatic External Defibrillators for Public Access Use: Recommendations for Specifying and Reporting Arrhythmia Analysis Algorithm Performance, Incorporation of New Waveforms, and Enhancing Safety.
-
Page 49: Accessories Specifications
Accessories specifications Battery M5070A category specifications battery type 9 VDC, 4.2 Ah, lithium manganese dioxide. Disposable, long-life primary cell. capacity When new, a minimum of 200 shocks or 4 hours of operating time at 77° F (25° C). (IEC 60601-2-4 2002) shelf life A minimum of 5 years from date of manufacture when stored and maintained (prior to insertion) - Page 50 The used pads may be contaminated with body tissue, fluid, or blood. Cut them off and dispose of them as infectious waste. Recycle the remaining cartridge components at an appropriate recycling facility in accordance with local regulations. HEARTSTART M5066A...
- Page 51 Configuration Overview The Philips HeartStart Defibrillator comes with a factory default configuration designed to meet the needs of most users. This configuration can only be changed by an authorized person using HeartStart Configure PDA software or Event Review software. This software is for use by trained personnel.
- Page 52 * A shock series begins when a shock is delivered after the HeartStart is turned on. A new shock series begins after a protocol pause. If shock series is configured for 2 or more, a new shock series also begins if the time since the previous shock exceeds the shock series interval setting. HEARTSTART M5066A settings default...
- Page 53 parameter settings protocol pause timer 0.5, 1.0, 1.5, (minutes) 2.0, 2.5, 3.0 NSA pause type • Standard NSA pause: HeartStart does not perform rhythm analysis during the NSA pause. • SMART NSA pause: HeartStart con- ducts background monitoring during the SMART NSA pause.
- Page 54 * If the shock series is configured to 2 or more, and a shock has been delivered as part of a series, the length of the first NSA pause within that shock series is determined by the protocol pause timer setting. Otherwise, the length of an NSA pause is determined by the NSA pause timer setting. HEARTSTART M5066A settings default 0.5, 1.0, 1.5,...
- Page 55 parameter settings CPR Coaching Yes, No adult ventilation instruction CPR Coaching Yes, No infant/child ventilation instruction CPR Coaching • 30:2 adult and compression:ventilation 30:2 infant/child ratio • 30:2 adult and 15:2 infant/child • 15:2 adult and 15:2 infant/child default default description Optional CPR Coaching includes rescue breaths at the rate determined by the CPR Coaching...
- Page 56 Notes HEARTSTART M5066A...
-
Page 57: Troubleshooting
In addition, it runs a pads self-test each time a pads cartridge is inserted. It alerts you if it finds a problem. See the Technical Reference Manual, available online at www.medical.philips.com, for a detailed discussion of the self- tests. - Page 58 There may be a problem with the pads cartridge. HEARTSTART M5066A possible cause removed. damaged. to the patient. contact with the patient's bare chest because of moisture or excessive hair.
- Page 59 HeartStart tells you: ... to stop all motion • The patient is being moved or jostled. • The environment is dry and movement around the patient is causing static electricity to interfere with ECG analysis. • Radio or electrical sources are interfering with ECG analysis.
- Page 60 If it fails again, do not use the defibrillator. If it passes, store the defibrillator within the recommended temperature range. • Contact Philips for service if needed. Remove the battery for five seconds then reinstall it to start the battery insertion self-test.
- Page 61 Additional technical information required for European conformity Electromagnetic conformity Guidance and manufacturer’s declaration: The HeartStart is intended for use in the electromagnetic environment specified in the tables below. The customer or user of the HeartStart should assure that it is used in such an environment. Electromagnetic emissions emissions test compliance...
- Page 62 Generally, AEDs are sometimes susceptible to interference generated by patient and/or responder motion in environments in which a high static electric field is present (e.g., low humidity, synthetic carpets, etc.). As a safety measure, Philips AEDs incorporate a patented method to sense possible corruption of the ECG signal by such interference and to respond by directing the user to stop all motion.
- Page 63 Recommended separation distances between portable and mobile RF communications equipment and the HeartStart The HeartStart is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the HeartStart can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the HeartStart as recommended below, according to the maximum output power of the communications equipment.
- Page 64 HeartStart takes <30 seconds from analyzing to ready-to-shock. After 200 shocks, the HeartStart takes <40 seconds from initial power-on to ready-to-shock. HEARTSTART M5066A Do not allow the pads to contact other electrodes or metal parts that are in contact with the patient.
- Page 65 Intentionally blank.
- Page 66 Intentionally blank.
- Page 67 Intentionally blank.
- Page 68 Intentionally blank.
- Page 69 Intentionally blank.
- Page 70 P O W E R S A V E Philips Medical Systems is part of Royal Philips Electronics REF: M5066-91901 L I F E Philips Medical Systems United States Philips Medical Systems 2301 Fifth Avenue, Suite 200 Seattle, WA, USA 98121...













